Fda Black Box Warning List 2024 – Now, the FDA has added to the list of potential uses for the drug due to one reported side effect. “There is a black box warning on this drug for ironically anaphylaxis,” Schreiber said. Also, not . An Iovance Biotherapeutics treatment employing a different type of cell has won accelerated FDA approval for advanced Amtagvi’s label carries a black box warning that the treatment can .
Fda Black Box Warning List 2024
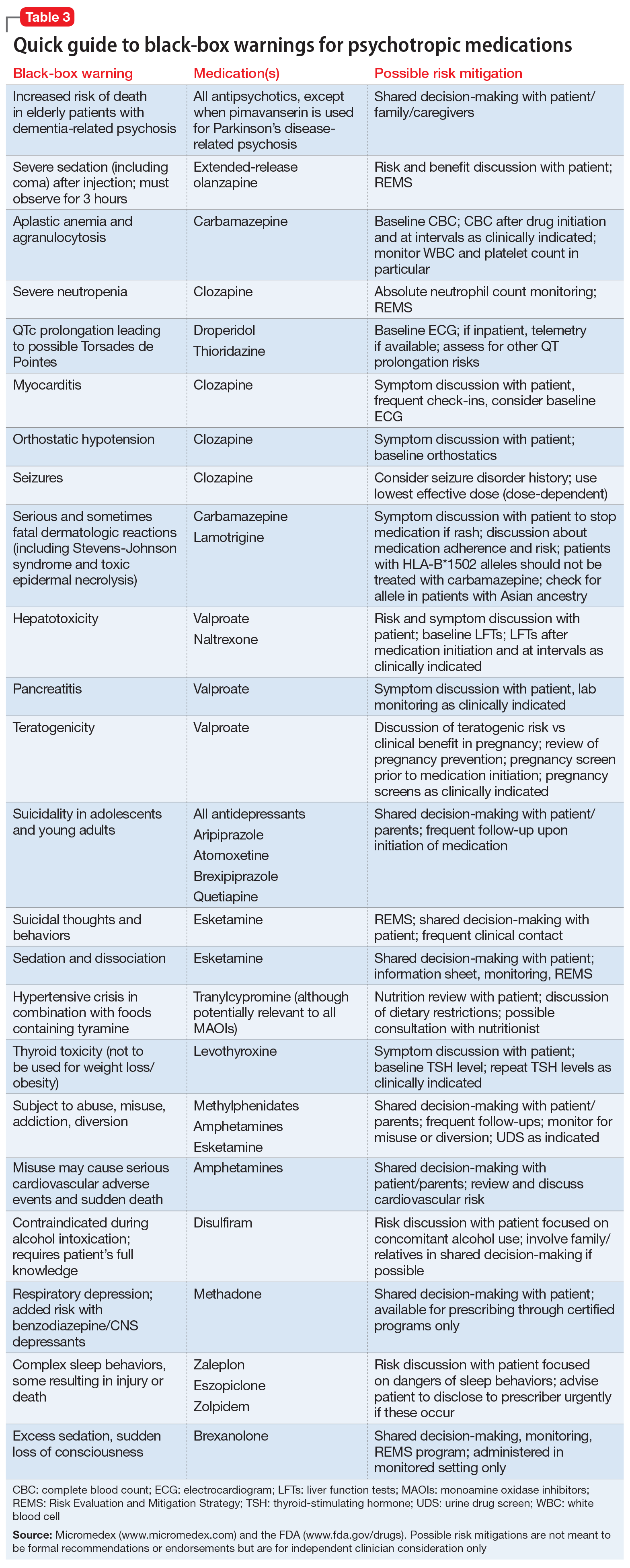
Source : fadic.netBlack box warnings: How they can improve your clinical practice
Source : www.mdedge.comRisks of Benzodiazepines | National Center for Health Research
Source : www.center4research.orgDrugs’ Links To Suicide Risk Draw Concern WSJ
Source : www.wsj.comSaunders Nursing Drug Handbook 2024: 9780443116070: Medicine
Source : www.amazon.comBoxed warning Wikipedia
Source : en.wikipedia.orgAll New 2024 Toyota Tacoma is Adventure Ready Out of the Box
Source : www.prnewswire.comBlack box warnings: Definition, medications, and more
Source : www.medicalnewstoday.comBlack box warnings: Definition, medications, and more
Source : www.medicalnewstoday.comFDA Issues Warning of Cancer Risk Tied to CAR T Therapies The
Source : www.nytimes.comFda Black Box Warning List 2024 Black Box Warning Medications: Comprehensive List Download: The Food and Drug Administration (FDA) issued a series of black box warnings in the 1990s for prokinetics and statistics — within each article and also list them in the resources section . The agency said that an increasing number of third-party test labs are generating testing data that is fabricated, duplicated from other submissions, or otherwise unreliable. .
]]>